What is pH Electrode Troubleshooting?
The pH electrode is the part of the pH meter that is in contact with the substance to be measured and is used to measure the electrode potential. When using a pH electrode, various problems may occur if the operation is not standardized, resulting in the inability to monitor the acidity and alkalinity of the water quality in real-time. Below we list several common problems and solutions.
Troubleshooting
Difficult to stabilize the reading, or the calibration is wrong
Reason: There are air bubbles in the electrode-sensitive membrane or gel. This phenomenon is more common in gel electrodes.
Solution: Shake the electrode down hard to drive away the air bubbles (shake the thermometer), and place the electrode vertically at ordinary times.
Why is displayed as 7.02 after being calibrated in a pH7.00 buffer?
Reason: The temperature of the buffer solution was around 20°C at that time, and its pH value changed with the change in temperature. 7.00 is the value of the buffer at 25°C, while at 20°C it should be 7.02. The pH probe electrode can automatically correct the influence of temperature on the buffer to ensure measurement accuracy.
After calibration with pH6.86 buffer, it will display 7.00
Reason: Wrong buffer group selected.
Solution: Reset to the correct buffer set.
In different samples, showing little variation
Reason: Poor contact between the electrode and the meter, or the electrode is broken.
Solution: After shutting down, reconnect the electrode and the meter or replace the electrode.
The slope obtained by calibration is higher than 100%
Reason: Buffer expired or contaminated.
Solution: Replace with the new buffer.
How should the electrodes be stored?
The basis for electrode storage is to make the preservation solution the same as the filling solution. For long-term storage, the buffer solution with pH=7 or pH=4 in 3mol/L KCl solution can be used for short-term storage.
Notice:
- The electrodes must not be placed dry.
- Do not store electrodes in distilled water.
The sample temperature is 10°C. What temperature does the meter display at this time? What can be done if the pH value at 25°C is required?
Explanation: The pH combination electrode shows the pH value at the current temperature. If a pH of 25°C is required, the temperature of the solution must be raised/lowered to 25°C before measurement. Sensors are electrochemical consumables and have a certain service life. Depending on the site conditions, the service life of the sensor is also different. Reasonable and scientific maintenance methods can make the service life of the sensor longer. Most of the problems that occur during on-site measurement are sensor problems.
Slow response time
Reason: Plastic gel-filled (non-refillable) electrodes are often favored for their low cost and low maintenance. However, they are much slower to respond than more expensive liquid-filled glass electrodes. Newer, functioning gel-filled electrodes can go from pH 7 to pH 4 in no more than 60 to 90 seconds, while glass refillable electrodes can take about half that time.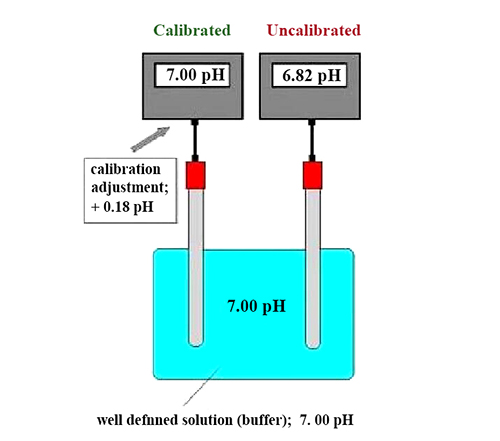
As electrodes age (especially gel-filled electrodes), they will slow down in response. A single-junction electrode used with an incompatible sample may give a very slow response for a short period of time because the electrode may have become irreversibly clogged. Using double-junction electrodes can prevent similar blockages in the future.
Note the response time during standardization with the standard buffer under pH calibration. In some cases, it is normal if your electrode responds quickly during calibration, but responds more slowly in your sample.
Solution: Dirty or dried-out high-temperature pH electrodes will be unresponsive. If rinsing with clean water isn't enough, try soapy water. Warm soapy water is effective on most organic matter, while low concentrations of acid are effective on most inorganic matter. After cleaning, a soaking period may be required to rehydrate the glass sensor bulb. A warm pH 4 buffer works well and is easy to confirm when hydration is complete.
Use plastic beakers during calibration or measurement. There seems to be an increased chance of encountering a slow response when mixing solutions using a magnetic stirrer. The rotation/friction of the stir bar in the plastic beaker will often cause the pH electrode to misbehave. Turning off or slowing down the stirrer and switching to a glass beaker, removing and reinserting the electrode from the solution may also help.
Static interference
Reason: Using a plastic beaker while stirring with a magnetic stir bar can create a static charge that can offset the reference potential and cause erratic pH readings.
Solution: Switch to a glass container or stop stirring and remove the electrode from the stirrer. Electrostatic charges can also occur in industrial applications of plastic pipes.
Electromagnetic noise
If the signal is not preamplified, the distance between the electrode and the controller must be kept to a minimum and less than 20 feet. Under no circumstances should cables used for unamplified signals be spliced. All wiring connections must be kept clean and dry. Electrode wires and any other low-voltage wires must be physically isolated in a separate conduit of at least six inches from the AC power wires, or erratic and inaccurate readings will result.

